UNMET NEEDS
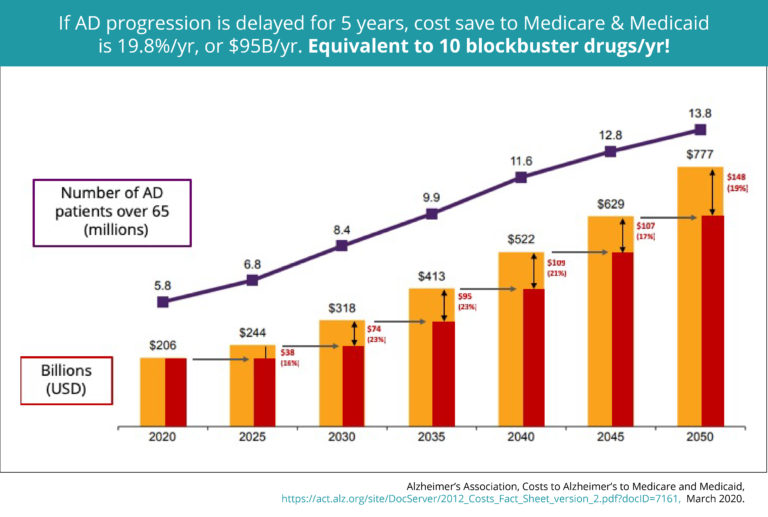
In 2019, 5.8 million Americans will suffer from Alzheimer's disease, and as many as 35 million people in the world will suffer from Alzheimer's disease. This graph shows that by 2050, an estimated 14 million Americans will develop Alzheimer's disease. A 2018 research report by the Alzheimer's Association pointed out that between 2000 and 2015, the number of people who died of Alzheimer's more than tripled, rising by 123%. On the other hand, this is in stark contrast to the 11% decline in deaths from heart disease, the leading cause of death in the United States.
Market Opportunity
By the time we reach 2050, it is projected to cost $777 billion.
Most of these patients’ amyloid-β protein level are unknown to their attending physicians since PET-scan costs $3000 to $4000 (without imbursement from CMS) and invasive spinal tap for CSF has low patients’ acceptance. Knowledge of amyloid-β protein level is clinically actionable. JAMA (April 2019) published a CMS-sponsored clinical trial where 11,409 patients with dementia completed PET-scan for amyloid-β. Results showed attending physicians changed management of 61% (6,959) of patients with known amyloid-β information.
In addition to sales to diagnostic companies or hospitals, another opportunity is companion diagnostics for pharmas in characterizing amyloid-β levels of subjects prior to enrollment for Alzheimer's Disease-related clinical trials. Recent interaction with IQVIA, the largest CRO worldwide, confirmed that this is an extremely urgent need for pharmas.